- Study protocol
- Open access
- Published:
Implementing evidence-based practices to improve primary care for high-risk patients: study protocol for the VA high-RIsk VETerans (RIVET) type III effectiveness-implementation trial
Implementation Science Communications volume 5, Article number: 75 (2024)
Abstract
Background
Patients with significant multimorbidity and other factors that make healthcare challenging to access and coordinate are at high risk for poor health outcomes. Although most (93%) of Veterans’ Health Administration (VHA) patients at high risk for hospitalization or death (“high-risk Veterans”) are primarily managed by primary care teams, few of these teams have implemented evidence-based practices (EBPs) known to improve outcomes for the high-risk patient population’s complex healthcare issues. Effective implementation strategies could increase adoption of these EBPs in primary care; however, the most effective implementation strategies to increase evidence-based care for high-risk patients are unknown.
The high-RIsk VETerans (RIVET) Quality Enhancement Research Initiative (QUERI) will compare two variants of Evidence-Based Quality Improvement (EBQI) strategies to implement two distinct EBPs for high-risk Veterans: individual coaching (EBQI-IC; tailored training with individual implementation sites to meet site-specific needs) versus learning collaborative (EBQI-LC; implementation sites trained in groups to encourage collaboration among sites). One EBP, Comprehensive Assessment and Care Planning (CACP), guides teams in addressing patients’ cognitive, functional, and social needs through a comprehensive care plan. The other EBP, Medication Adherence Assessment (MAA), addresses common challenges to medication adherence using a patient-centered approach.
Methods
We will recruit and randomize 16 sites to either EBQI-IC or EBQI-LC to implement one of the EBPs, chosen by the site. Each site will have a site champion (front-line staff) who will participate in 18 months of EBQI facilitation.
Analysis
We will use a mixed-methods type 3 hybrid Effectiveness-Implementation trial to test EBQI-IC versus EBQI-LC versus usual care using a Concurrent Stepped Wedge design. We will use the Practical, Robust Implementation and Sustainability Model (PRISM) framework to compare and evaluate Reach, Effectiveness, Adoption, Implementation, and costs. We will then assess the maintenance/sustainment and spread of both EBPs in primary care after the 18-month implementation period. Our primary outcome will be Reach, measured by the percentage of eligible high-risk patients who received the EBP.
Discussion
Our study will identify which implementation strategy is most effective overall, and under various contexts, accounting for unique barriers, facilitators, EBP characteristics, and adaptations. Ultimately this study will identify ways for primary care clinics and teams to choose implementation strategies that can improve care and outcomes for patients with complex healthcare needs.
Trial registration
ClinicalTrials.gov, NCT05050643. Registered September 9th, 2021, https://clinicaltrials.gov/study/NCT05050643
Protocol version
This protocol is Version 1.0 which was created on 6/3/2020.
Background
Patients who are at the highest risk for hospitalization (“high-risk patients”) are a heterogenous subset of patients who have significant multimorbidity and pose the most significant medical challenges within any healthcare organization [1]. These patients are at high risk for poor health outcomes and account for the majority of the Veterans Health Administration (VHA) healthcare costs [2], similar to other healthcare systems [3, 4]. Previous Medicare demonstrations, such as advanced primary care home models called Comprehensive Primary Care Plus (CPC +), have shown that caring for high-risk patients can be challenging despite financial alignment that promotes coordination of care delivery [5]. Primary care teams bear most of the responsibility in caring for complex patients; in VHA over 93% of high-risk Veterans are managed by general primary care teams, despite the availability of specialized primary care teams for patients with advanced health conditions [6]. Yet, complex, high-risk patients often do not receive the most effective evidence-based care within general primary care teams [7].
Many of the evidence-based practices (EBPs) have been ineffective in the management of high-risk patients due to the lack of EBPs that properly address multimorbidity— most EBPs focus on a single problem [8, 9]. However, there are a few EBPs that have shown to be effective for high-risk patients in geriatrics and other specialized settings, such as comprehensive assessments, individualized care plans, and care coordination among the multidisciplinary team members [10,11,12]. Evidence also supports patient-centered approaches to support self-management for high-risk patients with competing medical and self-care demands, including shared decision making and health coaching [12,13,14]. However, primary care teams have not implemented these EBPs widely [15, 16]. Despite the availability of these effective practices, the most effective implementation strategies to increase uptake of EBPs for high-risk patients in primary care are unknown. EBQI has been used successfully to implement complex EBPs in VA primary care, such as the patient-centered medical home model [17] and primary care-mental health integration [18, 19]. EBQI is, in fact, a bundle of implementation strategies that emphasizes a systematic approach to developing a researcher-clinical partnership that engages national, regional, and local-level senior organizational leaders and local QI teams in adapting and implementing EBPs in the context of prior evidence and local practice conditions [17]. Core elements of EBQI include engaging multi-level multidisciplinary stakeholders in evidence-based agenda setting (developing a “QI Roadmap”), training clinical champions in QI methods to meet agenda goals, and practice facilitation [17, 18, 20, 21]. The theoretical basis underlying EBQI elements includes theories of organizational change [22,23,24,25], clinical quality improvement, [26,27,28] complex adaptive systems [25], and diffusion of innovation [29]; each element can be mapped to documented implementation strategies [30]. However, beyond these core elements, EBQI initiatives have widely varied in the extent and types of interactions with implementation facilitators, specifically in practice facilitation and training. Some initiatives have combined EBQI core components with individual ongoing consultation [17, 20], which can require significant researcher and quality coordinator time and resources. Other VHA EBQI initiatives have used across-site learning collaboratives (i.e., depression collaborative care [18, 19] and opioid use disorder [31]), which may require fewer resources and impact a greater number of health professionals. While both variations have been effective, individual site-level consultation has never been empirically compared with learning collaboratives; implementers lack guidance on which of these strategies are effective in what setting.
Methods
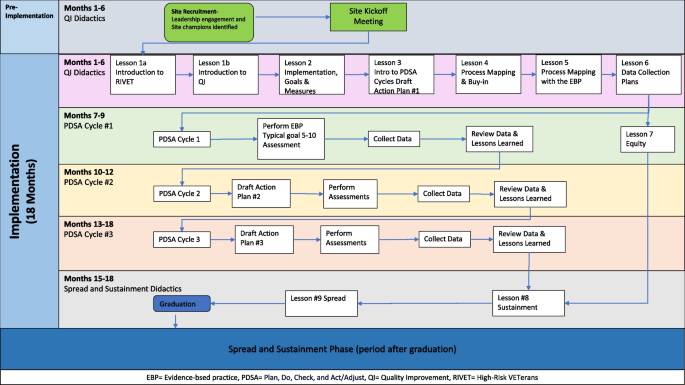
This study uses a mixed-methods type 3 hybrid implementation-effectiveness evaluation using a Concurrent Stepped Wedge design, evaluation of two separate interventions in different clusters, [32] to compare the two variants of EBQI aimed at increasing reach of the proposed EBPs (CACP or MAA). The Practical, Robust Implementation and Sustainability Model (PRISM) framework (Fig. 1) [33, 34] will guide the planning, implementation, and evaluation of the RIVET Program. The PRISM framework specifies contextual factors which align well with the components of our implementation strategies, and which will guide our evaluation of factors that influence Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) outcomes [34]. The Consolidated Framework for Implementation Research (CFRI) framework will identify implementation determinants, i.e., barriers and facilitators to implementation.
Evidence‑based practices (EBPs)
We selected two EBPs that guide primary care teams to identify modifiable needs among high-risk patients through standardized assessments and utilize the expertise of the multidisciplinary staff in the primary care team: Comprehensive Assessment and Care Plan (CACP) and Medication Adherence Assessment (MAA).
EBP Comprehensive Assessment and Care Plan (CACP) for high-risk patients
The CACP is an assessment that helps the primary care team to develop an individualized treatment plan based on identified needs for high-risk patients of any age [35]. It was adapted from the Comprehensive Geriatric Assessment (CGA), which has been shown in multiple randomized control trials (RCTs) to lead to improved outcomes for older adults with complex care needs [36, 37]. According to meta-analyses, the CGA has consistently led to improved outcomes for frail, older adults, such as decreased mortality (OR 0.86, 95% CI 0.75–0.98), decreased readmissions (OR 0.88, 95% CI 0.79–0.98), and decreased length of stay (1.63–40.7 days in intervention group vs 1.8–42.8 days in usual care) compared to those who did not receive the CGA [38,39,40].
While the CGA assesses several domains that are important for complex patients, some domains may not be broadly applicable to high-risk Veterans of all ages (i.e., nutrition, vision, hearing, continence). According to our analyses, half of high-risk Veterans are younger than 65 years old and have greater psychiatric comorbidities than older high-risk Veterans [6]. We added domains to specifically assess for modifiable risk factors that are common among high-risk Veterans (e.g., transportation assistance, health literacy, behavioral health symptoms, and coordination with non-VHA healthcare systems) [41,42,43]. The CACP screening questions are taken from standard sources, including the National Academy of Medicine Recommendations for High-Need Patients [44] and the Protocol for Responding to and Assessing Patients’ Assets, Risks, and Experiences (PRAPARE) [45].
The CACP prompts the primary care team member to explore identified risk factors further or to refer to another team member for an in-depth assessment if needed. After the assessment, the CACP is then used to guide the development and implementation of an individualized treatment plan that addresses the patient’s health-related needs in the context of the patient’s preferences [10]. The treatment plan can be coordinated and monitored through team huddles (consisting of the primary care provider, nurses, clerk) or monthly interdisciplinary team meetings.
EBP Medication Adherence Assessment (MAA)
High-risk Veterans often experience complicated medication regimens, potential side effects, and other known barriers to medication adherence [46]. Medication nonadherence represents a common problem among multimorbid patients [47], and is one of the largest contributors to preventable emergency department visits and hospitalizations among high-risk Veterans. Many approaches to improving medication adherence are limited by a focus on particular diseases rather than the entire medication regimen and by a focus on the healthcare providers’ point of view, rather than on patients’ goals and agency around medication taking [48].
A standardized Medication Adherence Assessment (MAA) guides primary care teams to assess for barriers and challenges to medication adherence through open-ended questions and enables primary care teams to understand high-risk patients’ goals and preferences and better impact medication-taking behaviors across the patients’ medication conditions [12, 13]. The MAA prompts the clinician to employ specific strategies around adherence and potentially use motivational interviewing or health coaching if the patient is ambivalent about taking a medication. Health coaching has emerged as an effective, patient-centered, collaborative approach to understand patient goals and preferences and enhance patients’ adherence to modifiable health behaviors [49]. Health coaching is a type of cognitive-based behavior change technique that employs motivational interviewing and goal-setting to guide the patient to change health behaviors, such as diet, exercise, and medication adherence [49]. Meta-analyses have shown that cognitive-based behavior change techniques (e.g., health coaching) to improve medication management are associated with an effect size of 0.34 (95% CI 0.23–0.46) on improved medication adherence [50].
EBQI Implementation strategies
The high-Risk VETeran (RIVET) Program will compare two variants of Evidence-Based Quality Improvement (EBQI)—practice facilitation through Individual Consultation (IC) or through Learning Collaboratives (LC) to determine which of the two is the most effective and less costly implementation strategy and explore which is better suited for which contexts.
Individual Consultation (IC)
Individual consultation, often described as coaching or supervision, is endorsed by implementation experts as an effective implementation strategy in and of itself. In RIVET, external facilitators from the study team will provide training to QI participants, who are front-line primary care staff, from an individual medical facility. The RIVET IC will provide regular individualized EBQI training and coaching to implement the EBP. Consultation allows experts to tailor complex skills and training to needs of the organization and to the QI participants, using active learning and providing practice opportunities [51]. It also provides QI participants with problem-solving skills and accountability [51]. Literature has demonstrated increased uptake and adherence to EBPs and increase sustainability with IC [51, 52].
Learning Collaboratives (LC)
Learning collaboratives are also widely used in healthcare settings and are an effective implementation strategy [30]. The RIVET learning collaboratives consists of external facilitators from the study team providing quality improvement training to QI participants from multiple sites in a group, and encourages interaction and collaboration among QI participants (e.g., providing feedback to each other). The effectiveness of learning collaboratives vary, but generally, they have demonstrated improvement in health professionals’ knowledge, problem-solving skills and attitude, and teamwork [53]. The mechanisms by which learning collaboratives may be effective include factors within an organization and factors between multiple organizations. In terms of factors within an organization, participation in learning collaboratives may increase staff confidence in using data to make decisions and to problem solve, increase accountability by making standards explicit, promote peer reflection, and facilitate teamwork, shared responsibility, and joint problem solving [53]. Mechanistic factors between organizations include normative pressure from peers, friendly competition, a platform for capacity building, and collaboration with other sites [53].
Site selection and eligibility
We will implement the EBPs at 16 primary care sites, targeting those with high ambulatory care-sensitive hospitalization rates. Each site will be implementing a single EBP. Implementation strategies are randomized by site. Site implementation consists of four overlapping cohorts of four sites (three randomized to LC and one to IC); all will undergo 18-months of RIVET facilitation (see Figure#1). Time periods without active implementation will serve as the usual care periods for EBQI strategies. Usual care sites will receive Office of Primary Care educational campaigns and dissemination of tools for high-risk patients among primary care teams. VHA regional leaders or VHA facility leaders will select the EBP for primary care sites to implement.
Setting
VHA primary care uses a multi-disciplinary patient-centered team-based approach (Patient-Aligned Care Teams; PACTs) where teams of health care professionals provide longitudinal care to a panel of patients [35]. Team members include a primary care provider, nurses, clerk, integrated mental health provider, social worker, and a pharmacist. Teams have access to multiple dashboards and reports for care management, including the Care Assessment Need (CAN) score [54], which describes the patient’s risk for future VA hospitalization or death by percentile.
EBQI activities
We first engaged regional and local multidisciplinary stakeholders to discuss implementation of each EBP, such as the target patient population and the clinical staff who might perform the assessment, developing a “QI Roadmap.” QI training and practice facilitation spans 18 months of video calls with site clinical champions, selected by their facility leadership. Meetings are led by a trained RIVET external facilitator and include structured QI didactics, designing Plan-Do-Study-Act cycles, coaching, review of data, and developing structured action plans (Fig. 1). The RIVET implementation team provides quarterly data reports and regularly discuss next steps with the champions.
The same structured QI didactics are utilized for both LC and IC groups (Fig. 1). The IC sites participate in individual meetings with the clinical champions every month, on average. Learning collaboratives consist of three EBQI-LC sites that participate in monthly meetings. All sites randomly assigned to EBQI-LC participate in the same learning collaborative regardless of EBP (CACP vs MAA), as both EBPs involve the same goal, the same target population, in the same clinical setting, similar to prior initiatives [17, 31, 55].
Data sources
Data sources include VHA Central Data Warehouse (CDW) administrative data, surveys to high-risk patients, surveys to Primary Care staff, key stakeholder interviews, implementation facilitation logs, time activity surveys, and site administrative documents.
EHR Administrative data
VHA CDW contains data on patient characteristics, outpatient encounters, provider types for each encounter, acute and inpatient care utilization, medication fill history, and Healthcare Effectiveness Data and Information Set (HEDIS) quality metric status. Health factor administrative data is generated by templates constructed for each EBP within the EHR. Managerial Cost Accounting (MCA) data will be utilized for the cost analyses.
High-risk patient surveys
Patient surveys will collect data on patient experiences among high-risk primary care patients at participating sites. Patient surveys will be mailed at the beginning and end of the 18-month active implementation phase to 500 randomly selected high-risk patients empaneled to primary care teams at each site, sampled cross-sectionally at each time period with replacement. Patient eligibility is based on the following criteria: a Care Assessment Need (CAN) score ≥ 90th percentile within the month prior to the sampling date; a visit with primary care within the last six months of the sampling date; and empanelment to the clinical champion team’s panel. When possible, patient experience and satisfaction questions were sourced from the Patient-Centered Medical Home (PCMH) version of the VHA Survey of Healthcare Experiences of Patients (SHEP), based on the Consumer Assessment of Healthcare Providers and Systems (CAHPS). Additional survey items measure direct impacts of specific EBPs (such as medication adherence), and items that may impact patient engagement in and benefit from EBPs, such as trust in their primary care provider (PCP). See Table 1 for details on included measures.
Clinical staff surveys
Clinical staff surveys will be administered site-wide, to assess factors that may influence update of RIVET EBPs at the clinic, 1) other tools, resources and practices used when managing high-risk patients, and 2) exposure to RIVET EBPs (post-implementation only), and 3) confidence with practices promoted by RIVET EBPs and with overall high-risk patient care. Most items were derived from previous VA primary care staff surveys, including those conducted for the purposes of evaluating staff experiences and approaches to high-risk patient care [65]. Electronic surveys will be sent to all primary care providers, nurses, and medical assistants on eligible teams at participating sites at the beginning and end of the 18-month active implementation phase. Eligible teams include general and women’s health primary care teams, as well as any ‘specialty’ primary care teams (e.g., geriatric primary care) that the site’s champion considers eligible for EBP spread. Clinician eligibility criteria includes being a member of at least one PACT teamlet at the RIVET site; being either a physician, physician assistant, nurse practitioner, registered nurse, licensed practical or vocational nurse, a medical assistant, or a health technician; providing direct patient care at the site; and working at the primary care site for at least three months.
Key stakeholder interviews
Guided by the Practical, Robust Implementation and Sustainability Model (PRISM) and Consolidated Framework for Implementation Research (CFIR) frameworks, we will conduct pre- and post- semi-structured qualitative telephone interviews with key middle managers (Primary Care Medical Director, Primary Care Nursing lead, Social Work lead, Integrated Mental Health lead, Pharmacy lead) and frontline clinicians who participate in EBQI and implementation activities. The interviews will assess readiness (inner context) and its subconstructs of leadership and engagement, available resources, and access to knowledge and information; implementation climate (inner context) and its subconstructs of relative priority and values; implementation process and its subconstructs of engaging key stakeholders and executing the implementation plan; characteristics of Individuals and its subconstruct of knowledge and beliefs about the intervention; and intervention characteristics and its subconstructs of relative advantage and complexity.
Implementation facilitation logs
We will use templated implementation facilitation logs to collect information about participants’ attendance at EBQI activities (including facilitation sessions and other meetings), participants’ role in RIVET, plan-do-study-act cycles, ‘real time’ adaptations to the EBPs, and barriers to implementation. The Implementation facilitators and coordinator will complete the implementation facilitation logs for both EBQI-IC and EBQI-LC sites after each meeting and any contact with implementation sites and leadership.
Time activity surveys
We will administer weekly time surveys to the RIVET implementation team which will capture RIVET staff time spent in various implementation activities. To assess time spent on RIVET implementation activities by site participants outside of facilitation sessions and other meetings with the RIVET implementation team, we will conduct brief monthly polls via Teams during facilitation sessions.
Periodic reflections
Using a semi-structured interview guide, a 30-min recorded monthly meeting will be conducted with the facilitation staff to elicit information and their overall impression of implementation progress and process. The meeting will document any implementation challenges and successes, adaptations, stakeholder engagement and relevant contextual issues. We will assess contextual factors, such as organizational readiness (leadership engagement, resources, access to knowledge and information) and anticipated barriers/facilitators at each site.
LC and IC site administrative documents
The site administrative documents include the QI roadmap for each EBP, site action plans developed by clinical champions, meeting minutes, written reports and presentations to leadership, and attendance records.
Measures
The Practical, Robust Implementation and Sustainability Model (PRISM) framework) [33, 34] will guide the planning, implementation, and evaluation of the RIVET Program. The PRISM framework specifies contextual factors which align well with the components of our implementation strategies, and which will guide our evaluation of factors that influence Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) outcomes [34]. We will use mixed methods to evaluate RE-AIM outcomes, EBP fidelity, implementation strategy fidelity, adaptations, costs, benefits, and value.
Reach
Reach is defined in this study as the proportion of high-risk patients on each study team’s panel that received one of the EBPs during the 18-month implementation period. See Table 2 for details on included reach measures. We define “high-risk patients” using a VHA-specific risk score called the Care Assessment Need score, previously developed and validated by VA through machine-learning to predict a patient’s risk for a VHA hospitalization or mortality [54]. We define ‘receipt’ as having the EBP assessment at least partially documented in the electronic health record (EHR). We will also examine the patient characteristics (sociodemographic, Elixhauser comorbidity score [66]) of eligible patients who did vs. did not receive the EBP.
Effectiveness
EBP effectiveness measures will include type of referrals or actions generated by each EBP, and whether they were completed, as measured by EHR template use, administrative consult data, and encounters. We will also use administrative EHR data to measure number of patient encounters with primary care team members (social work, pharmacist, nurse, integrated mental health), as an indicator of engagement of primary care teams for high-risk patient care. Impacts of RIVET on patient experience will be evaluated with a patient survey that includes measures (described above) of satisfaction and access to resources and support for caring for high-risk patients. We will also assess clinical performance metrics that are expected to be directly impacted by each EBP. For both EBPs, we will measure acute care utilization, such as emergency department (ED) visits, total acute and ambulatory care-sensitive (ACS)-related hospitalizations. For MAA, we will also measure adherence to medications for common chronic conditions, such as hypertension and diabetes. See Table 2 for details on included effectiveness measures.
Adoption
We will assess adoption by measuring number and proportion of staff trained on each EBP, and how many and which types of staff delivered each EBP (representativeness), using administrative training records and administrative clinical data for each EBP.
Implementation fidelity
We will assess implementation strategy fidelity using the EBQI fidelity assessment tool [55]. The fidelity assessment tool draws from data collected from key stakeholder interviews, implementation facilitation logs, administrative documents, and weekly time diaries (described below). We will apply criteria to rate sites as high, medium, or low fidelity on the EBQI elements. Using the implementation facilitation logs, we will assess participation in the EBQI activities by frontline providers, staff, and leadership.
Maintenance
We assess maintenance by the extent to which EBPs are implemented after practice facilitation ends (e.g., about 18 months). We also plan to study if the EBPs are spread to other primary care teams within the site and to other sites within the healthcare system.
Outcomes and data analyses
To analyze our primary outcome, receipt of each EBP among top 10th percentile high-risk patients during the 18-month implementation period (Reach), we will model uptake of both practices in our Concurrent Stepped Wedge Design as a multilevel hierarchical model with a repeated cross-sectional data structure in which sites are followed over time. In this design, the data structure includes patients at level 1 (where Reach, the main outcome of interest, in measured), nested within time at level 2, and nested within sites at level 3. The main predictors will be site implementation strategy assignment (EBQI-IC vs. EBQI-LC vs Usual Care) over time (measure as the number of quarter). We will first describe differences in trends using unadjusted analyses, then add covariates for patient characteristics (i.e., age, sex, Elixhauser comorbidity score [66]) and site-level covariates (i.e., number of unique patients served in primary care, rural vs urban). Secondary analyses of Reach will include models by key patient subgroups, including those hospitalized in the 90 days prior to the quarter examined, and models with an interaction term for assigned implementation strategy by EBQI fidelity level (as defined above), to examine how implementation strategy fidelity may have impacted Reach.
To analyze our secondary outcomes of care processes, patient experiences, provider experiences, and performance measures (Effectiveness), we will first model unadjusted trends in outcomes over time for each EBP, by site implementation strategy assignment. We will then model outcomes using two or three-level (depending on whether the metric is measured at the patient or site level) hierarchical models based on the concurrent Stepped Wedge Design using repeated cross-sectional data, adjusted for the same covariates included in the models for our primary outcome. In models of medication adherence, we will add covariates for medication regimen complexity, as indicated by total number of classes of medication prescribed for the condition of interest, and patient co-pay status. If we find differences in outcomes, we will perform mediation analyses that consider how Reach and EBP fidelity may have mediated outcomes [67].
To assess differences in adoption between EBQI-IC and EBQI-LC sites, we will use bivariate analyses to compare the number and proportion of staff trained on each EBP, and how many and which types of staff delivered each EBP. To assess differences in implementation strategy fidelity among sites, we will compare the number of sites with high, medium, and low fidelity on each element of EBQI, as well as overall fidelity to EBQI. Similarly, we will compare number of sites with overall high, medium, or low EBP fidelity among EBQI-IC vs EBQI-LC sites.
We will qualitatively assess the impact of contextual factors on implementation, using a matrix analysis approach [68] to explore a priori themes based on the interview guides, but also allow for emergent themes. Specifically, we will code and analyze interview data for core elements of the EBQI implementation strategy and for contextual features, intervention characteristics, and implementation infrastructure guided by the PRISM framework [33]. Two trained qualitative analysts will construct and validate the codebook [69]. Using this codebook, one analyst will code all interviews, and a second qualitative analyst will review all coding. After generating a report for all codes, they will use a matrix analysis approach [68] to populate a participant-by-theme matrix and create site level summaries for each theme to facilitate comparisons between EBQI-IC and EBQI-LC sites. To ensure rigor, summaries will also be reviewed, compared with the original data, and discussed by at least two analysts to reach consensus for any discrepancies. Finally, we will link this qualitative matrix with site-level implementation strategy and EBP fidelity measures to compare how specific contextual features, intervention characteristics, and implementation infrastructure impacted fidelity.
Return-On-Investment (ROI) Analyses
We will conduct a budget impact analysis to inform the sustainability of each EBP. Following the VHA recommendation to evaluate cost of projects [70], we will identify the relevant costs associated with implementation of the EBP, the EBP itself, and the consequences of the EBP (e.g., healthcare utilization). Using a micro-costing approach [71], we will collect costs for EBQI-IC and EBQI-LC sites, measuring: 1) implementation costs as one-time costs to develop the intervention; and 2) intervention and downstream costs as costs that would be incurred by facilities adopting the EBPs (e.g., site participants; RIVET implementation team members’ time spent in training, meetings and preparing for meetings; staff time performing the intervention). To capture RIVET implementation team staff time spent in various implementation activities, data will be collected through the implementation facilitation log, administrative documents, and weekly time surveys. For both EBPs, clinical staff will document the estimated time spent to complete EBP through an EHR template. Finally, we will identify the costs of healthcare utilization which may be impacted by the implementation of these EBPs from the VA administrative data, such as change in outpatient utilization (e.g., primary care, social work, mental health, pharmacy) and inpatient utilization. This will be done by estimating the excess cost for patients exposed to the EBP compared to a control group of unexposed patients using multivariable regression models to control for measured confounders. We will use generalized linear models for continuous data and two-part models for semi-continuous data with distributional families chosen to best fit the data. If preliminary data reveals that medication adherence changed with MAA implementation, we will also include changes in pharmacy costs.
We will measure whether EBPs were maintained during each sites’ sustainment period (time period following the 18-month active implementation period), how EBPs spread within the original sites and to new sites, and what factors are associated with maintenance and spread. We will also assess adaptations made to the EBPs in response to changing VHA context.
For sustainment and spread, we will continue to assess receipt of each EBP among top 10% CAN score patients (Reach) longitudinally during the sustainment period by measuring 6 and 12 months after the active implementation phase. We will incorporate Adoption measures to assess spread within the implementation site and in new sites by measuring how many additional staff were trained on each EBP, proportion of staff trained on each EBP, and which types of healthcare staff delivered each EBP (representativeness). We will compare EBQI-IC and EBQI-LC sites to determine which implementation strategy is most effective for sustaining the EBPs. Using the matrix analysis approach described above, we will analyze qualitative interview data to explore the role of contextual factors on sustainment, and the “fit” between context, intervention, and implementation strategy on sustainability.
Discussion
The RIVET project aims to implement two evidence-based assessments to improve the management of the high-risk patient population within VHA primary care using two EBQI strategies. This work will add to a much needed body of literature evaluating the effectiveness of different approaches to EBQI to implement EBPs within primary care [1]. It is the first study to compare the effectiveness of EBQI conducted with individual sites (EBQI-IC) vs conducted with groups of sites as a learning collaborative (EBQI-LC) and to compare which implementation strategy is most effective under various contexts accounting for unique barriers, facilitators, and adaptations. Additionally, RIVET will provide evidence on which of the two strategies is the most cost-efficient strategy. Comparing EBQI-LC and EBQI-IC will allow our VHA primary care leaders to tailor the implementation strategy to the primary care context in preparation for widespread implementation. While our project focuses on EBPs for high-risk patients, we anticipate that this comparison of EBQI strategies can inform those implementing EBPs with other primary care patient populations.
The EBPs aim to systematically identify modifiable risk factors within primary care for patients with complex needs—enabling primary care to provide comprehensive and holistic care. Furthermore, by selecting the most effective, less burdensome, and less costly implementation strategy ensures greater buy-in from clinical leadership interested in offering advanced primary care and frontline staff who are often overwhelmed with clinical demands and chronic staffing shortages.
We anticipate several potential challenges to optimal implementation. The major challenge for the RIVET project is that, as with any project embedded in pragmatic healthcare system operations, it is vulnerable to national and local VHA contextual factors. Specifically, success of the project can be compromised by VHA staffing changes within the sites and study teams. In addition, since primary care teams are tasked with a wide variety of care, new health system initiatives and external circumstances (e.g., pandemic-induced changes in care delivery) can unexpectedly compete with high-risk patient care priorities. In addition, the active implementation period requires 18-month engagement from clinical champions to learn EBQI practices and to properly use the EBP in their routine care of high-risk patients. Finally, RIVET EBQI IC and LC sessions will be held virtually. Although most provider training has moved to virtual modalities post-Covid, the best methods to keep staff engaged may vary over time and setting. The RIVET project will not only implement EBP tools that will help better manage high-risk Veterans at 16 VA sites but will provide the tools and evidence on the best implementation strategies for primary care staff at VHA working to improve high-risk patient care and primary care delivery.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ACS:
-
Ambulatory care-sensitive
- CACP:
-
Comprehensive Assessment and Care Planning
- CAHPS:
-
Consumer Assessment of Healthcare Providers and Systems
- CAN:
-
Care Assessment Need
- CFIR:
-
Consolidated Framework for Implementation Research
- CGA:
-
Comprehensive Geriatric Assessment
- CPC + :
-
Comprehensive Primary Care Plus
- CDW:
-
Central Data Warehouse
- EBP:
-
Evidence-based practice
- EBQI:
-
Evidence-Based Quality Improvement
- ED:
-
Emergency department
- EHR:
-
Electronic health record
- HEDIS:
-
Healthcare Effectiveness Data and Information Set
- IC:
-
Individual coaching
- LC:
-
Learning collaborative
- MAA:
-
Medication Adherence Assessment
- MCA:
-
Managerial Cost Accounting
- OPC:
-
Office of Primary Care
- PACT:
-
Patient-Aligned Care Team
- PCMH:
-
Patient-Centered Medical Home
- PCP:
-
Primary care provider
- PRAPARE:
-
Protocol for Responding to and Assessing Patients’ Assets, Risks, and Experiences
- PRISM:
-
Practical, Robust Implementation and Sustainability Model
- QUERI:
-
Quality Enhancement Research Initiative
- RCT:
-
Randomized control trial
- RE-AIM:
-
Reach, Effectiveness, Adoption, Implementation, and Maintenance
- RIVET:
-
High-RIsk VETerans
- ROI:
-
Return-On-Investment
- SHEP:
-
Survey of Healthcare Experiences of Patients
- VHA:
-
Veterans’ Health Administration
References
Hempel S, et al. Evidence-based quality improvement: a scoping review of the literature. J Gen Intern Med. 2022;37(16):4257–67.
Zulman DM, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771.
Marengoni A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–9.
Smith SM, et al. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ. 2012;345:e5205.
Higgins TC, O’Malley AS, Keith RE. Exploring and overcoming the challenges primary care practices face with care management of high-risk patients in CPC+: a mixed-methods study. J Gen Intern Med. 2021;36(10):3008–14.
Chang ET, et al. Use of general primary care, specialized primary care, and other veterans affairs services among high-risk veterans. JAMA Netw Open. 2020;3(6):e208120.
Hebert PL, et al. Patient-centered medical home initiative produced modest economic results for Veterans Health Administration, 2010–12. Health Aff (Millwood). 2014;33(6):980–7.
Skou ST, et al. Multimorbidity. Nat Rev Dis Primers. 2022;8(1):48.
Boyd CM, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24.
Boult C, Wieland GD. Comprehensive primary care for older patients with multiple chronic conditions: “Nobody rushes you through.” JAMA. 2010;304(17):1936–43.
O’Toole TP, et al. Tailoring Outreach efforts to increase primary care use among homeless veterans: results of a randomized controlled trial. J Gen Intern Med. 2015;30:886–98.
Poitras M-E, et al. What are the effective elements in patient-centered and multimorbidity care? A scoping review. BMC Health Serv Res. 2018;18(1):446.
Tinetti M, et al. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32(2):261.
Jones A, et al. A national evaluation of homeless and nonhomeless veterans’ experiences with primary care. Psychol Serv. 2017;14(2):174.
Midboe AM, et al. Implementing motivational interviewing in primary care: the role of provider characteristics. Transl Behav Med. 2011;1(4):588–94.
Stockdale S, et al. What do patient-centered medical home (PCMH) teams need to better manage care for their patients at high-risk for hospitalization or mortality? In: in society of general internal medicine annual meeting. Washington, DC: Society of General Internal Medicine; 2019.
Rubenstein LV, et al. Evidence-based quality improvement: a method for helping managed primary care practices become patient centered. J Gen Intern Med. 2014;29:589–97.
Rubenstein LV, et al. Using evidence-based quality improvement methods for translating depression collaborative care research into practice. Fam Syst Health. 2010;28:91–113.
Chang ET, et al. Use of general primary care, specialized primary care, and other veterans affairs services among high-risk veterans. JAMA Netw Open. 2020;3(6):e208120.
Yano EM, et al. Cluster randomized trial of a multilevel evidence-based quality improvement approach to tailoring VA patient aligned care teams to the needs of women Veterans. Implement Sci. 2016;11(1):101.
Hamilton A, et al. Engaging multilevel stakeholders in an implementation trial of evidence-based quality improvement in va women’s health primary care. Transl Behav Med. 2017;7(3):478.
Lukas CV, et al. Transformational change in health care systems: an organizational model. Health Care Manage Rev. 2007;32(4):309–20.
Ovretveit J. Improvement leaders: what do they and should they do? A summary of a review of research. Qual Saf Health Care. 2010;19(6):490–2.
Øvretveit J. Understanding the conditions for improvement: research to discover which context influences affect improvement success. BMJ Qual Saf. 2011;20 Suppl 1(Suppl_1):i18–23.
Crabtree BF, et al. Primary care practice transformation is hard work: insights from a 15-year developmental program of research. Med Care. 2011;49 Suppl(l):28–35.
Kaplan HC, et al. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13–20.
King G, et al. A framework of operating models for interdisciplinary research programs in clinical service organizations. Eval Program Plann. 2008;31(2):160–73.
Berwick DM. The science of improvement. JAMA. 2008;299(10):1182–4.
Greenhalgh T, et al. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82(4):581–629.
Powell BJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21.
Chang ET, Oberman RS, Cohen AN, Taylor SL, Gumm E, Mardian AS, et al. Increasing access to medications for opioid use disorder and complementary and integrative health services in primary care. J Gen Intern Med. 2020;35:918-26.
Lyons VH, Li L, Hughes JP, Rowhani-Rahbar A. Proposed variations of the stepped-wedge design can be used to accommodate multiple interventions. J Clin Epidemiol. 2017;86:160–7.
McCreight MS, et al. Using the Practical, Robust Implementation and Sustainability Model (PRISM) to qualitatively assess multilevel contextual factors to help plan, implement, evaluate, and disseminate health services programs. Transl Behav Med. 2019;9(6):1002–11.
Glasgow RE, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019;7:64.
Elsawy B, Higgins KE. The geriatric assessment. Am Fam Physician. 2011;83(1):48–56.
Reuben DB, et al. A randomized trial of comprehensive geriatric assessment in the care of hospitalized patients. N Engl J Med. 1995;332(20):1345–50.
Garrard JW, et al. Comprehensive geriatric assessment in primary care: a systematic review. Aging Clin Exp Res, 2019;32(2):197–205.
Ellis G, et al. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. 2011;343:d6553.
Ellis G, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;9(9):006211.
Stuck AE, et al. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342(8878):1032–6.
Hatef E, et al. Integrating social and behavioral determinants of health into patient care and population health at Veterans Health Administration: a conceptual framework and an assessment of available individual and population level data sources and evidence-based measurements. AIMS Public Health. 2019;6(3):209–24.
Zullig LL, et al. A systematic review of conceptual frameworks of medical complexity and new model development. J Gen Intern Med. 2016;31(3):329–37.
Cornell PY, et al. Embedding social workers in veterans health administration primary care teams reduces emergency department visits. Health Aff (Millwood). 2020;39:603. https://doi.org/10.1377/hlthaff.2019.01589.
Long P, et al. Effective care for high-need patients: opportunities for improving outcomes, values, and health. In: in NAM special publication leadership consortium for a value & science-driven health system. Washington, DC: National Academy of Medicine; 2017.
National Association of Community Health Centers. PRAPARE: Protocol for Responding to and Assessing Patients' Assets, Risks, and Experiences. 2019; Research and Data:[Available from: http://www.nachc.org/research-and-data/prapare/. Cited 2019 Dec 9
Hanlon J, et al. Suboptimal prescribing in older inpatients and outpatients. J Am Geriatr Soc. 2001;49(2):200.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97.
Viswanathan M, et al. Medication therapy management interventions in outpatient settings: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(1):76–87.
Gierisch J, Hughes J, Edelman D. The Effectiveness of Health Coaching. Washington (DC): Department of Veterans Affairs (US); 2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK487700/.
Easthall C, Song F, Bhattacharya D. A meta-analysis of cognitive-based behaviour change techniques as interventions to improve medication adherence. BMJ Open. 2013;3(8):e002749.
Nadeem E, Gleacher A, Beidas RS. Consultation as an implementation strategy for evidence-based practices across multiple contexts: unpacking the black box. Adm Policy Ment Health. 2013;40(6):439–50.
Waltz TJ, et al. Choosing implementation strategies to address contextual barriers: diversity in recommendations and future directions. Implement Sci. 2019;14(1):42.
Zamboni K, et al. How and under what circumstances do quality improvement collaboratives lead to better outcomes? A systematic review. Implement Sci. 2020;15(1):27.
Wang L, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368–73.
Stockdale SE, et al. Assessing fidelity to evidence-based quality improvement as an implementation strategy for patient-centered medical home transformation in the Veterans Health Administration. Implement Sci. 2020;15(1):18.
Department of Veterans Affairs. SHEP Patient Centered Medical Homes (PCMH) Short Form (VA 10-1465-5). Department of Veterans Affairs. 2021. https://omb.report/icr/202102-2900-017/doc/109373600. Accessed 7 Nov 2024.
Voils CI, et al. Initial validation of a self-report measure of the extent of and reasons for medication nonadherence. Med Care. 2012;50(12):1013–9.
Zulman DM, et al. Effects of intensive primary care on high-need patient experiences: survey findings from a veterans affairs randomized quality improvement trial. J Gen Intern Med. 2019;34(Suppl 1):75–81.
Rosland AM, et al. Effectiveness of a health coaching intervention for patient-family dyads to improve outcomes among adults with diabetes: a randomized clinical trial. JAMA Netw Open. 2022;5(11):e2237960.
Jensen RE, et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res. 2015;24(10):2333–44.
Chew LD, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23(5):561–6.
Billioux A, Verlander K, Anthony S, Alley D. Standardized Screening for Health-Related Social Needs in Clinical Settings: The Accountable Health Communities Screening Tool. NAM Perspectives. Discussion Paper. National Academy of Medicine; 2017. https://nam.edu/wpcontent/uploads/2017/05/Standardized-Screening-for-Health-Related-Social-Needs-in-Clinical-Settings.pdf. Accessed 10 July 2024.
Anderson GO, Thayer CE. Loneliness and social connections: A national survey of adults 45 and older. Washington, DC: AARP Foundation; 2018.
Deverts DJ, et al. Comparing the effectiveness of Family Support for Health Action (FAM-ACT) with traditional community health worker-led interventions to improve adult diabetes management and outcomes: study protocol for a randomized controlled trial. Trials. 2022;23(1):841.
Meredith LS, et al. Can using an intensive management program improve primary care staff experiences with caring for high-risk patients? Fed Pract. 2021;38(2):68.
Elixhauser A, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Baron R, Kenny D. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173.
Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855–66.
Miles M, Huberman A. Qualitative data analysis: an expanded sourcebook. Thousand Oaks, CA: Sage; 1994.
Veteran's Administration Health Economics Resource Center. What are the steps to conduct economic analyses for implementation research? 2019 August 7, 2019; Available from: https://www.herc.research.va.gov/include/page.asp?id=implementation-steps. Cited 2019 December 3
Liu CF, et al. Organizational cost of quality improvement for depression care. Health Serv Res. 2009;44(1):225–44.
Acknowledgements
We would like to acknowledge Kelsey Cummings, MS and Emily Wong, MPH for their contribution to the implementation of the project; Bridget Kranke, MSSA, LSW for assisting with editing; and the VA Office of Primary Care (OPC), Geriatrics and Extended Care (GEC), Patient-Centered Care and Cultural Transformation (OPCC&CT), Primary Care Improvement and Innovation and the primary care leads from Veterans Integrated Services Networks (VISNs) 9, 10, 12 for their advice and support.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs, or the US government, or other affiliated institutions.
Funding
This project is funded by the VA Quality Enhancement Research Initiative (QUERI) program, project #: QUE-20–018.
Author information
Authors and Affiliations
Contributions
Contributions to the creation and design of the funded project includes AMR, SS, and EC. Development of the project design at implementation stage includes EJ, AMR, SS, AR, MW, NT, AH, and EC. All listed authors contributed to writing/editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The RIVET project was determined to qualify as non-research conducted under the authority of VHA Office of Primary Care, as it was designed and implemented with the intent to improve internal patient care at VHA and not conduct systematic research to advance scientific knowledge base. In accordance with VHA policies and guidelines, this program is considered as non-research by IRB (Subcommittee on Human Studies) of the VHA Greater Los Angeles Healthcare System Research Service (691/151) which is authorized to determined projects as non-research activities for which additional IRB oversight is not required, as defined per VHA Handbook 1058.05 in the section “Officials Authorized to Provide Documentation of VHA Program Office Non-Research Operations Activities” and later updated in Sect. 5a of the VHA Program Guide 1200.21.
Consent for publication
Not applicable.
Competing interests
Author EC has a consultancy with Behavioral Health Services, Inc. The remaining authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jimenez, E.E., Rosland, AM., Stockdale, S.E. et al. Implementing evidence-based practices to improve primary care for high-risk patients: study protocol for the VA high-RIsk VETerans (RIVET) type III effectiveness-implementation trial. Implement Sci Commun 5, 75 (2024). https://doi.org/10.1186/s43058-024-00613-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43058-024-00613-9